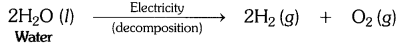
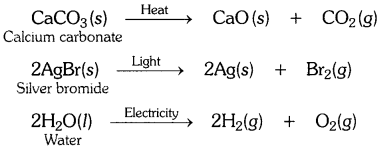
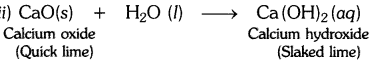EVENTS CONVENT HIGH SCHOOL
16/11/2021 CLASS- 9 SESSION 2021-22
SUBJECT : SOCIAL SCIENCE (HISTORY)CHAPTER-3 NAZIMS AND THE RISE OF HITLER
______________________________________
Question-1
Describe the problems faced by the Weimar Republic.
Solution:
The problems faced by the Weimar Republic were as follows:
- The Weimar constitution had Inherent drawbacks making the Weimar Republic fragile.
- One was proportional representation which made it difficult for any party to get a majority leading to coalition governments.
- Article 48, which gave the President the power to rule by decree, suspend civil rights and to impose an emergency.
People lost confidence in a democratic parliamentary system.
- The Weimar Republic was not received well by the people because of the terms it was forced to accept at Versailles at the end of the First World War. It was a harsh and humiliating treaty that Germany had to accept with the Allies. Many people held the Weimar Republic responsible for the defeat in the war and for accepting the terms of the Treaty of Versailles,
- In 1923 Germany refused to pay reparation payments and the French occupied its leading industrial area, the Ruhr, to claim their coal. Germany offered passive resistance and printed paper currency. With too much paper currency in circulation, the value of the German mark fell. The situation is called hyperinflation.
- The Weimar Republic had to face another economic crisis. The USA Withdrew her support when Wall Street Exchange crashed in 1929. German economy was the worst hit by the economic crisis. Workers lost their jobs or were paid reduced wages. The number of unemployed reached 6 million. The economic crisis created a feeling of fear among the people.
Question-2
Discuss why Nazism became popular in Germany by 1930.
Solution:
In 1919 Adolph Hitler took over the German Workers’ Party and called it the Nazi Party, giving birth to Nazism in Germany.
During the Great Economic Depression Nazism became very popular. The Nazi Propaganda which was very unique helped in making Nazism very popular. In his powerful speeches, Hitler promised to build a strong nation, restore the dignity of the Germans and provide employment for all. Numerous public meetings were held by the Nazi Party to instil unity among the people.
The red banners, the Nazi salute, and the rounds of applause attracted the people and Nazism became very popular. The Meetings projected Hitler as a saviour of Germany. The German people who were shattered after the First World War believed him.
Question-3
What are the peculiar features of Nazi thinking?
Solution:
The peculiar features of Nazi thinking are as follows.
- Nazi ideology was the same as Hitler’s worldview. According to this there was no equality between people but only a racial hierarchy. In this view blond, blue-eyed Nordic German Aryans were at the top called ‘desirables’ while Jews (undesirables) were placed at the lowest rung. Hitler’s racism was influenced by thinkers like Charles Darwin and Herbert Spencer. The Nazi argument was simple: ‘The strongest race would survive, the weak ones would perish’.
- Hitler believed in Lebensraum or living space. New territories had to be conquered to increase the living space.
- Nazis wanted a society of pure and healthy Nordic Aryans. It meant that even those Germans who were seen as impure or abnormal had no right to live. Under the Euthanasia Programme, the Nazi condemned to death many Germans, who were mentally or physically unfit.
- As soon as Hitler came to power he tried to eliminate the undesirables and the gypsy. The Nazis proceeded to realise their murderous racial ideals.
- Jews remained the worst sufferers in Nazi Germany. They were called ‘undesirables’. Hitler’s hatred for Jews was based on pseudoscientific theories of race, which held conversion was no solution. They should be completely eliminated.
Question-4
Explain why Nazi propaganda was effective in creating a hatred for Jews.
Solution:
Films were made to create hatred for the Jews. The film, ‘The Eternal Jew’, showed the Jews with flowing beards and dressed in kaftans. The Jews were referred to as vermin, rats, and pests. Nazi propaganda compared the Jews to rodents.
Orthodox Jews were stereotyped as killers of Christ and money lenders. Stereotypes about Jews were even popularised through maths classes. Children were taught to hate the Jews. The Nazi propaganda against the Jews was so effective that people felt anger and hatred surge inside them when they saw someone who looked like a Jew.
Question-5
Explain what role women had in Nazi society. Return to Chapter 1 on the French Revolution. Write a paragraph comparing and contrasting the role of women in the two periods.
Solution:
In Nazi Germany, boys were told to be aggressive and steel-hearted, girls were told that they had to become good mothers and rear pure-blooded Aryan children. Girls had to protect the purity of German race. They had to look after their homes and Nazi values had to be taught to the children.
Women who produced ‘desirable children’ were awarded. They got better treatment in hospitals, and got concessions in shops, on theatre tickets and railway fares. Honour crosses were awarded to women. A bronze cross was awarded to women for four children, silver cross for six children and gold for eight or more children.
Question-6
In what ways did the Nazi state seek to establish total control over its people?
Solution:
The Nazi state tried to establish total control over its people. Special forces were created to control the society, in the way Nazis wanted. Apart from SA or the Storm Troopers, and regular police, who wore a green uniform, these included the Gestapo (secret state police) and SS (the protection squads), criminal police, and the Security Service (SD). They were given extra-constitutional powers, that gave the Nazi state its reputation as the most dreaded criminal state. People were tortured in Gestapo chambers and sent to concentration camps. People were arrested without any legal procedures.