EVENTS CONVENT HIGH SCHOOL
08/01/2022 CLASS- 9 SESSION 2021-22
SUBJECT :SCIENCE
CHAPTER-4
STRUCTURE OF ATOM
______________________________________
Textbook – Page 47
Question 1. What are canal rays?
Answer: Canal rays are positively charged radiations which led to the discovery of positively charged sub-atomic particle called proton.
Question 2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer: The atom will be electrically neutral as one – ve charge balances one + ve charge.
Textbook – Page 49
Question 1. On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer: According to Thomson’s model of an atom
(i) An atom consists of a positively charged sphere and the electrons are embedded in it,
(ii) The negative and positive charges are equal in magnitude. So the atom is electrically neutral.
Question 2. On the basis of Rutherford’s model of an atom, which sub-atomic particle is present in the nucleus of an atom?
Answer: As per Rutherford’s model of an atom, the protons which are positively charged are present in the nucleus of an atom.
Question 3. Draw a sketch of Bohr’s model of an atom with three shells.
Answer: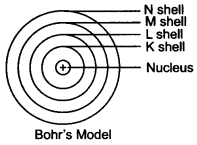
Question 4. What do you think would be the observation if the a-particle scattering experiment is carried out using a foil of a metal other than gold?
Answer: On using any metal foil, the observations of the a-particle scattering experiment would remain the same as all atoms would have same structure.
Textbook – Page 49
Question 1. Name the three sub-atomic particles of an atom.
Answer: The sub-atomic particles of an atom are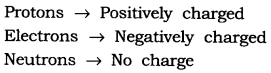
Question 2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer: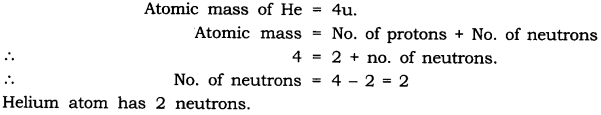
Textbook – Page 50
Question 1. Write the distribution of electrons in carbon and sodium atoms.
Answer: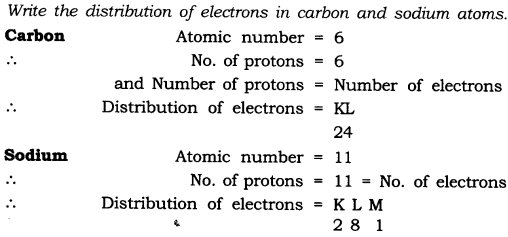
Question 2. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer: K shell can hold 2 electrons and L shell can hold 8 electrons.When both the shells are full, there will be (8 + 2) 10 electrons in the atom.
Text Book
Question 1. Compare the properties of electrons, protons and neutrons.
Answer: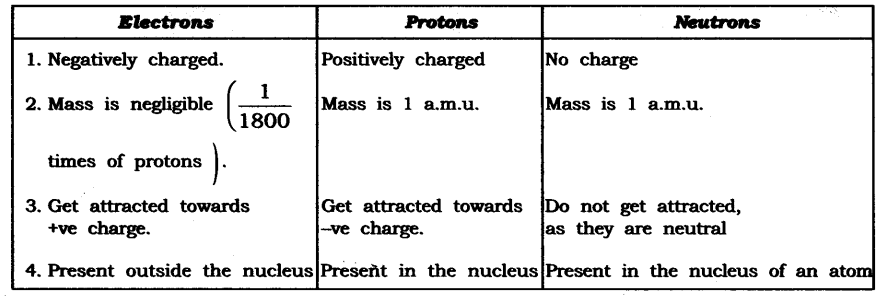
Question 2. What are the limitations of J.J. Thomson’s model of the atom?
Answer: According to J.J. Thomson’s model of an atom, the electrons are embedded all over in the positively charged spheres. But experiments done by other scientists showed that protons are present only in the centre of the atom and electrons are distributed around it.
Question 3. What are the limitations of Rutherford’s model of the atom?
Answer: According to Rutherford’s model of an atom the electrons are revolving in a circular orbit around the nucleus. Any such particle that revolves would undergo acceleration and radiate energy. The revolving electron would lose its energy and finally fall into the nucleus, the atom would be highly unstable. But we know that atoms are quite stable.
Question 4. Describe Bohr’s model of the atom.
Answer: Bohr’s model of the atom
(1) Atom has nucleus in the centre.
(2) Electrons revolve around the nucleus.
(3) Certain special orbits known as discrete orbits of electrons are allowed inside the atom.
(4) While revolving in discrete orbits the electrons do not radiate energy.
(5) These orbits or shells are called energy levels.
(6) These orbits or shells are represented by the letters K, L, M, N or the numbers n = 1, 2, 3, 4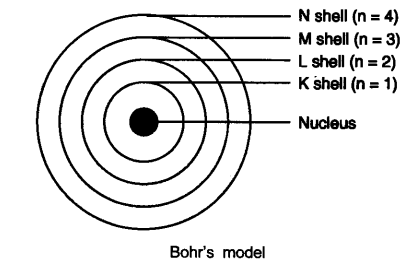
Question 5. Compare all the proposed Bohr’s models of an atom given in this chapter.
Answer: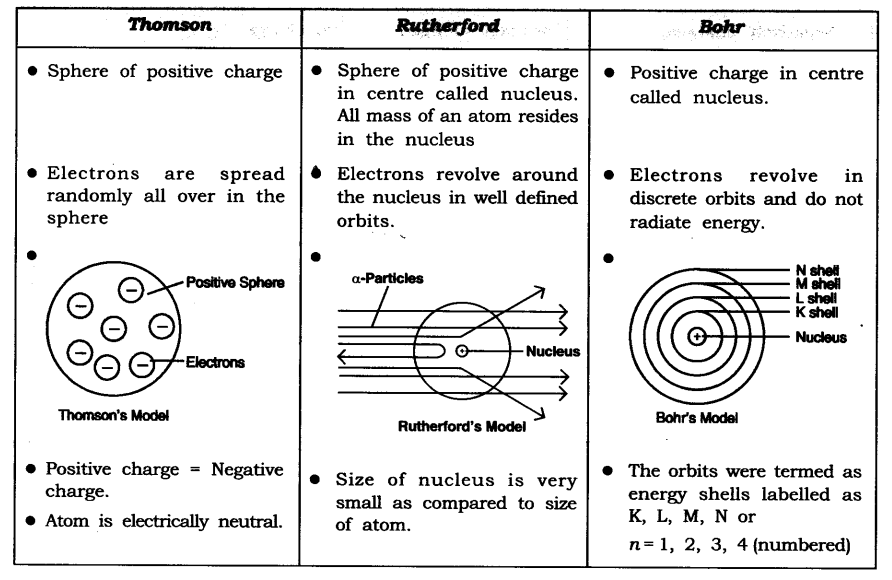
Question 6. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore,it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer: (a) False (b) False
(c) True (d) False
Question 7. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus (c) Proton
(b)Electron (d)neutron
Answer: (a) Atomic nucleus
Question 8.Isotopes of an element have
(a) the same physical properties (c) different number of neutrons
(b)different number of neutrons (d) different atomic numbers.
Answer: (c) different number of neutrons
Question 9. Number of valence electrons in Ct ion are :
(a) 16 (b) 8
(c) 17 (d) 18
Answer: (b) 8
Question 10. Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8 (b) 8, 2, 1
(c) 2, 1, 8 (d) 2, 8, 1
Answer: (d) 2, 8, 1
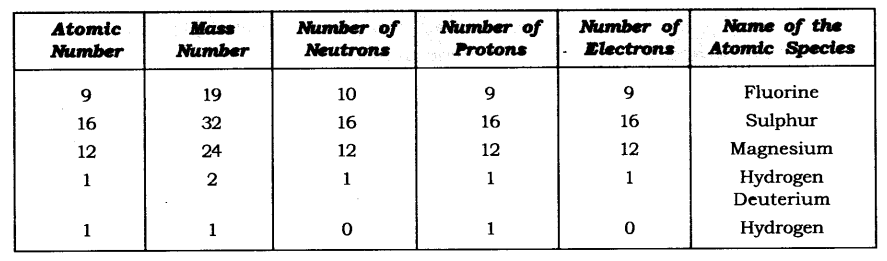
Question 11. Complete the following table.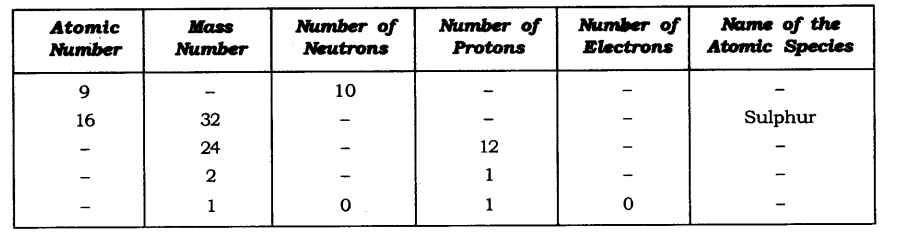
Answer: